Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

McGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kimuro, Shingo; Ishidera, Takamitsu
Journal of Nuclear Science and Technology, 60(12), p.1586 - 1594, 2023/12
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology) and Tb
and Tb measured by gel electrophoresis techniques
measured by gel electrophoresis techniquesNakano, Sumika*; Marumo, Kazuki*; Kazami, Rintaro*; Saito, Takumi*; Haraga, Tomoko; Tasaki-Handa, Yuiko*; Saito, Shingo*
Environmental Science & Technology, 55(22), p.15172 - 15180, 2021/11
Times Cited Count:5 Percentile:33.8(Engineering, Environmental)Humic acid (HA) can strongly complex with metal ions to form a supramolecular assembly via coordination binding. However, determining the supramolecular size distribution and stoichiometry between small HA unit molecules constituting HA supramolecule and metal ions has proven to be challenging. Here, we investigated the changes in the size distributions of HAs induced by Cu and Tb
and Tb ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu
ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu and Tb
and Tb complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
 ; Effect of matrix oxidation
; Effect of matrix oxidationOgawa, Toru; Verrall, R. A.*; Hj.Matzke*; P.G.Lucata*
Solid State Ionics, 49, p.211 - 216, 1991/00
Times Cited Count:4 Percentile:33.14(Chemistry, Physical)no abstracts in English


 by solid state EMF technique
by solid state EMF techniqueNakamura, Akio; Fujino, Takeo
Journal of Nuclear Materials, 149(1), p.80 - 100, 1987/01
Times Cited Count:44 Percentile:95.51(Materials Science, Multidisciplinary)no abstracts in English


 at relatively small deviation from stoichiometry between 600 and 1400
at relatively small deviation from stoichiometry between 600 and 1400 C
CNakamura, Akio; Fujino, Takeo
Journal of Nuclear Materials, 140, p.113 - 130, 1986/09
Times Cited Count:17 Percentile:83.74(Materials Science, Multidisciplinary)no abstracts in English


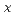 (0≦x≦0.1)
(0≦x≦0.1)Ugajin, Mitsuhiro
Journal of Nuclear Science and Technology, 20(3), p.228 - 236, 1983/00
Times Cited Count:19 Percentile:85.98(Nuclear Science & Technology)no abstracts in English


 solid solutions
solid solutionsUgajin, Mitsuhiro
Journal of Nuclear Materials, 110, p.140 - 146, 1982/00
Times Cited Count:24 Percentile:88.74(Materials Science, Multidisciplinary)no abstracts in English
 -NdO
-NdO

 and PuO
and PuO -YO
-YO

 solid solutions
solid solutions; ; ;
Journal of Nuclear Science and Technology, 19(8), p.681 - 683, 1982/00
Times Cited Count:2 Percentile:43.66(Nuclear Science & Technology)no abstracts in English
 powders
powdersShiba, Koreyuki; ; Ugajin, Mitsuhiro
Journal of Nuclear Materials, 96(3), p.255 - 260, 1981/00
Times Cited Count:6 Percentile:62.77(Materials Science, Multidisciplinary)no abstracts in English
Fujino, Takeo; Tagawa, Hiroaki
Journal of Physics and Chemistry of Solids, 34(10), p.1611 - 1626, 1973/10
Times Cited Count:10no abstracts in English
Kumagai, Yuta; Fidalgo, A. B.*; Jonsson, M.*
no journal, ,
Radiation-induced oxidative dissolution of nuclear fuel is anticipated in the geological repository of spent fuel and after severe accidents, where the fuel would be in direct contact with water. Therefore, understanding of the oxidative dissolution of UO is indispensable to estimate radioactive release. This study has addressed effects of hyperstoichiometry of UO
is indispensable to estimate radioactive release. This study has addressed effects of hyperstoichiometry of UO and adsorption of organic compounds on the process. Comparison between hyperstoichiometric UO
and adsorption of organic compounds on the process. Comparison between hyperstoichiometric UO and stoichiometric UO
and stoichiometric UO demonstrated that the reactivity of UO
demonstrated that the reactivity of UO approaches that of UO
approaches that of UO by the H
by the H O
O reaction. The result indicates formation of hyperstoichiometric surface layer during the oxidative dissolution. Phthalic acid, which was used as a model compound, suppressed the U dissolution by
reaction. The result indicates formation of hyperstoichiometric surface layer during the oxidative dissolution. Phthalic acid, which was used as a model compound, suppressed the U dissolution by  -ray irradiation but had little involvement in the H
-ray irradiation but had little involvement in the H O
O reaction, in spite of adsorption exceeding 1 molecule/nm
reaction, in spite of adsorption exceeding 1 molecule/nm . The results suggest an involvement of radical intermediate derived from phthalic acid in the surface reaction.
. The results suggest an involvement of radical intermediate derived from phthalic acid in the surface reaction.